Why Quality Matters
Introduction
In the fast-paced world of clinical research everyone is always rushing to get studies completed so that they can get their product into the marketplace and available for prescribers and patients. Studies have also become more complex and costly. The pressures driving the need for top-line results often leads to a reduced focus on the quality of the research activities. We often see an attention to clinical quality only when a significant issue arises during the conduct of a study, or at the end of a study when a Sponsor is preparing or a regulatory submission.
In this paper, 2Richards discuss the rationale for organizations to devote resource to clinical quality. We have found that presenting the discussion on why quality matters can facilitate discourse with Sponsors’ Executive Management and move the engagement of quality from a late stage, reactive activity to the development of a proactive, embedded mindset which benefits the organization.
Please note that these are our opinions based on our experience. It is the responsibility of the reader to review appropriate regulations and guidelines to determine appropriate actions to be taken regarding clinical trial conduct.
Three reasons why it is important to conduct high quality clinical trials.
Aside from the obvious statement that we should always try to conduct all our activities well, there are three fundamental reasons why clinical trials should always be of conduct to high quality:
1. Because it is a regulatory requirement.
The conduct of clinical trials is highly regulated by national legislation, international guidance, and regulatory standards. For example, it is clearly stated in ICH Guideline for Good Clinical Practice E6(R2) that there is an expectation for quality to be part of the clinical development process from start to finish:
“The sponsor should implement a system to manage quality throughout the design, conduct, recording, evaluation, reporting and archiving of clinical trials. The quality management system [QMS] should use a risk-based approach” [ICH E6(R2) GCP 5.0]
Accordingly, this is the expectation of international regulators responsible for reviewing clinical trial data and study conduct as part of a regulatory submission for marketing authorization.
2. To ensure protection of Participants’ rights
Clinical trials are experiments which are conducted to determine whether a proposed treatment is safe and will work to improve human health. Research participants (subjects/patients) agree to become part of these experiments and we owe them a duty of care through conducting high quality work so that they are appropriately informed of potential risks and are able to make informed consent decisions.
As the study continues, we need to ensure that accurate emerging safety data identified through this experimental work are promptly provided to regulators, ethics committees, Investigators and as required, study participants.
We are obligated to minimize risk to participants, (e.g., clear dosing instructions) and to protect their personal information (e.g., GDPR, HIPAA, etc.), by using effective, quality-controlled processes to control chain of custody of their data.
3. To ensure integrity of study data
Finally, it is essential to have confidence in the results of the study (positive or negative) so that appropriate business decisions can be made regarding the product’s lifecycle.
A basic tenet of scientific methodology is that a controlled experiment should be documented in such a way that it is reproducible and that study results can be relied upon to draw valid conclusions. Consistent, quality-assured processes are required to ensure this.
Business reasons for having a Clinical Quality Management System (QMS)
Often overlooked are some key benefits to investing in a clinical quality management system, for example:
1. Because it helps manage risk
Successfully conducting clinical research is an extremely complex and costly process requiring an interconnected web of experts and skilled professionals. Accordingly, many Sponsors contract out some or all key activities that are needed for the trials. However, as we are all aware, the Sponsor always retains overall responsibility for the conduct of the work, as outlined in ICH E6(R2):
“A sponsor may transfer any or all of the sponsor's trial-related duties and functions to a CRO, but the ultimate responsibility for the quality and integrity of the trial data always resides with the Sponsor.” [ICH E6(R2) GCP 5.2.1]
“The sponsor should ensure oversight of any trial-related duties and functions carried out on its behalf.” [ICH E6(R2) GCP 5.2.2 Addendum]
There are higher risks inherent with outsourcing an activity since the Sponsor has less direct control. Delays in communication or miscommunication between parties may also result in work not being performed as intended or to the requisite regulatory requirements. Any outsourced activity requires oversight by the Sponsor to allow rapid identification, reporting, assessment, and mitigation of emergent risks.
This is where experienced quality assurance personnel are required to evaluate risks to the development program and advise management accordingly.
2. Because it helps avoid re-work
Clinical trials are reliant on the reproducibility of robust processes and having consistent, standardized approaches. Failure in these critical processes frequently results in either total or partial re-work with attendant increased in resources, cost, and time.
Examples of processes failures which can have significant implications on the outcome of a study, or the success of a regulatory filing include:
Poor vendor oversight (resulting, for example, in need to re-monitor Sites).
Lack of TMF maintenance.
Inadequate computer system validation.
Need for database unlocks/relocks.
A well-implemented QMS allows for such issues to be identified promptly and corrected, so reducing the likelihood of significant re-work with its subsequent business implications.
3. Because sooner is better than later
In the face of positive trial data, Sponsors frequently rush to implement new processes and procedures that will meet regulatory expectations during a potential regulatory inspection. This approach incurs all the expense without any of the benefits of a QMS being in place during the study when it would have had positive operational impact. In addition, this workload comes at a time when the organization is already busy with preparing regulatory submission(s) and preparing for potential market launch.
This last-minute approach is not acceptable to EMA inspectors who expect studies to have been conducted using appropriate quality systems. This lack of a robust quality system will likely result in GCP inspection findings up to and including questioning the integrity of data being submitted to support a marketing application.
Inspectional Focus on Clinical Quality Management Systems
The implementation of revision two to ICH E6 in June 2017 emphasized the importance of implementing quality management early in the clinical trial environment. This will be further expanded with the upcoming revision three to the ICH E6. As can be seen from the following case studies, failure to implement a robust QMS in a timely manner can lead to severe consequences:
Case Study 1: Novel antibiotic for a significant unmet need
FDA GCP inspections were conducted at several Investigator Sites and the Sponsor which generated no findings. Subsequently the NDA was approved for this product to be marketed in the US. The Sponsor also submitted an MAA application to the EU and the EMA conducted GCP inspections at several Investigator Sites and of the Sponsor. Their inspectors issued 3 Critical and 7 Major findings and concluded that the integrity of study data was considered unreliable.
As a result of these findings, the Sponsor was required to re-monitor all Investigator Sites and advise EMA of findings, at significant cost. Approval of the product in the EU was delayed by over a year, resulting in delays to accessibility to physicians and patients and loss of sales for the Sponsor.
Case Study 2: Pulmonary arterial anti-hypertensive
The Sponsor was granted US marketing approval by FDA. However, after submitting an MAA to the EU, GCP inspections were conducted by EMA at Investigator Sites and the Sponsor facility. These inspections identified issues considered to constitute a serious breach of GCP.
Despite the Sponsor appealing the decision and endeavoring to defend conduct of the study, the EU marketing application was rejected.
Case Study 3: Vaccine
Following FDA Site GCP inspections no findings were identified. However, subsequent EMA GCP inspections at 3 Investigator Sites resulted in 6 Critical and 37 Major findings being reported. This triggered additional GCP inspections at the Sponsor and the CRO that had been contracted by the Sponsor.
The outcome of these activities resulted in the Sponsor also receiving 2 Critical and 7 Major findings and the GCP Inspectors deemed study data unacceptable to support the filing claims.
In all these examples having a robust Quality Management System built into the clinical development process from the beginning would have identified potential quality issues in a timely manner. This would have allowed prompt assessment, reporting and mitigation of the risk and resulted in better outcomes.
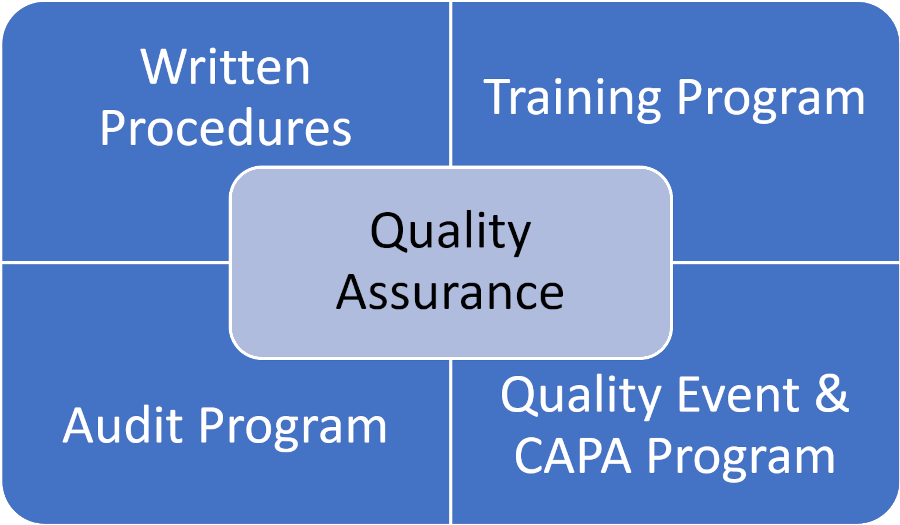
The Four Core Components of a Clinical Quality Management System (QMS)
There are four core components that interact and constitute an appropriate QMS in the clinical research space, as illustrated in the following diagram:
1. Written Procedures
Written procedures, in the form of Policies, Standard Operating Procedures and Guidelines are essential to provide common instructions to personnel and ensure consistent performance. ICH E6(R2) is clear in the requirement:
“The sponsor is responsible for implementing and maintaining quality assurance and quality control systems with written SOPs to ensure that trials are conducted, and data are generated, documented (recorded), and reported in compliance with the protocol, GCP, and the applicable regulatory requirement(s).” [ICH E6(R2) GCP 5.1.1]
The QMS also extends to study-related documentation such as Study Manuals and Plans which include the specific details required by staff to perform study-related activities.
We often see missing, expired, or incomplete SOPs at Sponsors and CROs in addition to a plethora of study plans created by CROs that are often unnecessarily complex, inconsistent, and can even be contradictory. It is important that the process for developing and maintaining all these documents is simple and efficient so that they remain relevant and do not cause issues with the quality of the deliverables they cover.
2. Training Program
Once a Sponsor or CRO has the relevant procedural documents in place it is essential that the relevant staff are trained to these documents. It is an expectation of ICH E6(R2) that only qualified, appropriately trained staff work in clinical research:
“Each individual involved in conducting a trial should be qualified by education, training, and experience to perform his or her respective task(s).” [ICH E6(R2) GCP 2.8]
“Risk reduction activities include defining roles and responsibilities, adhering to SOPs, and training in processes and procedures.” [ICH E6(R2) GCP 5.0.4]
Furthermore, it is an expectation that there is documented training for each individual prior to them conducting the work assigned. Sponsors and CROs often struggle to provide evidence of the timeliness of all training, both to SOPs and study-specific procedures especially, for example, in the context of access to computerized systems.
3. Audit Program
ICH E6(R2) is clear on the importance of an independent assessment of study-related activities:
“The purpose of a sponsor's audit, which is independent of and separate from routine monitoring or quality control functions, should be to evaluate trial conduct and compliance with the protocol, SOPs, GCP, and the applicable regulatory requirements.” [ICH E6(R2) GCP 5.19.1]
“The sponsor's audit plan and procedures for a trial audit should be guided by the importance of the trial to submissions to regulatory authorities, the number of subjects in the trial, the type and complexity of the trial, the level of risks to the trial subjects, and any identified problem(s).” [ICH E6(R2) GCP 5.19.3]
We often see Sponsors schedule audits toward or at the end of study enrollment as a check-box exercise. This, in our view, is a wasteful activity. A risk-based audit program to evaluate vendors, clinical sites, systems, and internal processes is required to ensure that the clinical development process is robust. This is something Sponsors must take responsibility for. It is not seen as best practice, for example, to have the CRO contracted for monitoring to also audit the Sites they are monitoring.
4. Quality Event & CAPA Program
As we have noted several times, clinical development is extremely complex. It is inevitable that not everything will go perfectly; even the regulators acknowledge this. However, there is an expectation that the QMS has processes in place to identify and investigate issues quickly, correct where possible and, importantly, to prevent reoccurrence:
“Noncompliance with the protocol, SOPs, GCP, and/or applicable regulatory requirement(s) by an investigator/institution, or by member(s) of the sponsor's staff should lead to prompt action by the sponsor to secure compliance.” [ICH E6(R2) GCP 5.20.1]
“When significant noncompliance is discovered, the sponsor should perform a root cause analysis and implement appropriate corrective and preventive actions.” [ICH E6(R2) GCP 5.20.1 Addendum]
“If required by applicable law or regulation the sponsor should inform the regulatory authority(ies) when the noncompliance is a serious breach of the trial protocol or GCP.” [ICH E6(R2) GCP 5.20.2]
The Quality Event and Corrective Action and Preventive Action program does not have to be complex, but it should be well understood by staff and utilized to capture those instances when issues arise so that appropriate assessment and actions can be taken.
Conclusion
We rely on appropriately conducted, well-controlled clinical studies to further advance pharmaceutical medicine. These studies are complex, involving many clinical investigators, vendors, and myriad systems to produce results for us to analyze. It is essential that all clinical research activities are conducted to expectations and high quality so that the results of these analyses can be trusted. Regulators have recognized that the volume of data that they need to inspect to assure quality is so large that it now makes more logical sense to shift focus to the quality systems that Sponsors, CROs, and sites have implemented to assure that the work is conducted to plan, subject welfare is protected, and study data can be relied upon. Fundamental to the quality system is the concept of Quality by Design (QbD) which focuses on embedding quality into the clinical trial processes from the start of a development program so that risks can be identified and mitigated in a timely manner to allow the science under study to be relied upon.
At 2Richards we have decades of experience in developing and implementing GCP quality management systems for dozens of organizations of varying sizes and stages of development. If you would like to learn how we can help, please see more at 2Richards.com.
Note: This article is also available as a downloadable, printable PDF by clicking here.